Kinetics, Catalysis, and Radioactivity: Fundamental Scientific Concepts Explained
Understand kinetics in scientific research
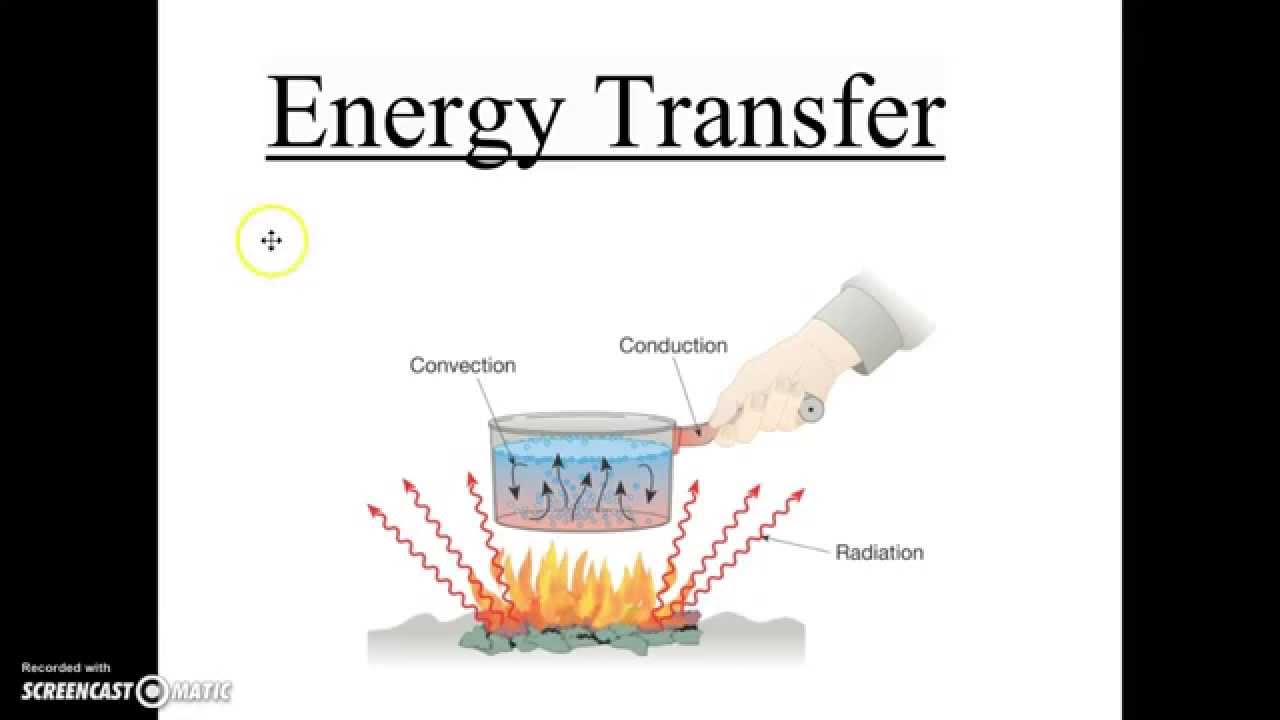
Kinetics stand as a cornerstone of scientific understanding, especially in the fields of chemistry and physics. At its core, kinetics examine the rates at which chemical processes occur and the factors that influence these rates. This branch of science provide crucial insights into how and why reactions happen, offer a window into the dynamic nature of matter.
The study of kinetics begins with reaction rates — measurements of how rapidly reactants transform into products. These rates depend on several key factors:
- Concentration of reactants
- Temperature of the system
- Presence of catalysts
- Surface area of solid reactants
- Pressure (for gas phase reactions )
Kinetic theory extend beyond chemical reactions to explain the behavior of gases. This theory posit that gas particles move arbitrarily, perpetually collide with each other and their container walls. The average kinetic energy of these particles instantly relate to temperature — higher temperatures mean fasting move particles.
Kinetic energy in physical systems
Kinetic energy — the energy of motion — play a vital role across scientific disciplines. In physics, the formula k = ½MV² express this energy, where m represent mass and v represent velocity. This simple equation underpin countless physical phenomena, from the motion of planets to the behavior of subatomic particles.
Kinetic molecular theory help explain many observable properties of matter. For instance, diffusion — the gradual mixing of substances due to random molecular motion — demonstrate kinetic principles in action. When you place a drop of food color in water, the color slow spread throughout the liquid as molecules move haphazardly from areas of high concentration to areas of lower concentration.
In biochemistry, enzyme kinetics examine how these biological catalysts accelerate reactions in live organisms. The Michaelis Lenten equation describe how reaction rates vary with substrate concentration, provide a mathematical framework for understand these critical biological processes.

Source: gosciencegirls.com
Catalysis: accelerate chemical transformations
Catalysis represent one of chemistry’s well-nigh powerful concepts, enable reactions that would differently proceed overly slow to be practical. A catalyst increase a reaction’s rate without being consumed in the process, make it a reusable tool in chemical transformations.
How catalysts work
Catalysts function by provide an alternative reaction pathway with a lower activation energy — the energy barrier that reactants must overcome to form products. By lower this barrier, catalysts make reactions more energetically favorable without change the overall energy difference between reactants and products.
Catalysts come in several forms:
-
Homogeneous catalysts
Exist in the same phase as the reactants, typically dissolve in solution -
Heterogeneous catalysts
Exist in a different phase than the reactants, ofttimes as solids interact with liquid or gas reactants -
Enzymes
Serve as biological catalysts, with extremely specific active sites that bind to particular substrates -
Autocatalysis
Occur when a reaction product catalyzes its own formation
Industrial applications of catalysis are vast and economically significant. The Haber-Bosch process use an iron catalyst to produce ammonia from nitrogen and hydrogen, enable the manufacture of fertilizers that feed billions of people ecumenical. Catalytic converters in vehicles employ platinum, palladium, and rhodium to transform harmful exhaust gases into less toxic substances.

Source: gosciencegirls.com
Catalysis in sustainable chemistry
Catalysis play a central role in green chemistry initiatives, offer paths to more environmentally friendly chemical processes. By enable reactions under milder conditions with fewer byproducts, catalysts reduce energy consumption and waste generation. Researchers presently explore novel catalytic materials, include metal organic frameworks and nanoparticles, to develop more efficient and selective catalytic systems.
Photocatalysis — use light energy to drive chemical reactions — represent a frontier in sustainable chemistry. Titanium dioxide and other photocatalysts can harness sunlight to break down pollutants in water and air, offer promise solutions for environmental remediation.
Radioactivity: nuclear transformations and applications
Radioactivity, discover by Henri Becquerel in 1896, basically change our understanding of atomic structure. This phenomenon occurs when unstable atomic nuclei release energy and particles through radioactive decay, transform into more stable configurations.
Types of radioactive decay
Radioactive elements undergo several distinct decay processes:
-
Alpha decay
Involve the emission of an alpha particle (two protons and two neutrons ) reduce the atomic number by 2 -
Beta decay
Occur when a neutron converts to a proton, emit an electron (beta particle )and an antineutrino -
Gamma radiation
Involve the release of high energy photons as excited nuclei transition to lower energy states -
Positron emission
Happen when a proton converts to a neutron, release a positron and a neutrino -
Electron capture
Occur when a nucleus capture an orbital electron, convert a proton to a neutron
The rate of radioactive decay follows a predictable pattern describe by half life — the time require for half of a radioactive sample to decay. Half lives range from fractions of a second to billions of years, depend on the isotope. This predictability make radioactive isotopes valuable as date tools in archaeology, geology, and other fields.
Applications of radioactivity
Despite public concerns about radiation hazards, radioactivity serve numerous beneficial purposes in modern society:
Medical applications
Include both diagnostic and therapeutic uses. Radioisotopes like technetium 99 m enable medical imaging through techniques such as single photon emission compute tomography (sspent) Radiotherapy employ cautiously control radiation doses to destroy cancer cells while minimize damage to healthy tissue.
Industrial applications
Leverage radioactive properties for non-destructive testing. Radiography use gamma rays to inspect welds and structural components for hidden flaws. Gauges contain radioactive sources measure thickness, density, and moisture content in manufacturing processes.
Energy production
Through nuclear fission presently generate roughly 10 % of the world’s electricity. Nuclear power plants use control chain reactions, typically involve uranium 235, to produce heat that generate steam to drive turbines.
Scientific research
Benefits from radioactive tracers that track biological processes, chemical reactions, and environmental pathways. Carbon 14 date has revolutionized archaeology by enable accurate age determination of organic materials up towell-nighh 50,000 years old.
The intersection of kinetics, catalysis, and radioactivity
These three scientific concepts — kinetics, catalysis, and radioactivity — intersect in fascinating ways, create synergies that drive scientific innovation.
Nuclear kinetics and chain reactions
Nuclear kinetics describe the rates and mechanisms of radioactive processes. In nuclear reactors, control chain reactions depend on precise kinetic relationships between neutron production and absorption. Moderators like water or graphite slow neutrons to thermal energies, increase the probability of uranium 235 fission and sustain the chain reaction.
The kinetics of radioactive decay influences applications from nuclear medicine to waste management. Understand decay rates and pathways help medical professionals calculate appropriate radioisotope doses for patients and guide engineers in design storage systems for radioactive waste.
Radio catalysis: radiation induced catalysis
Radiation can initiate or enhance catalytic processes through radio catalysis. High energy radiation create reactive species like free radicals that can catalyze chemical reactions. This phenomenon find applications in polymer synthesis, where radiation initiate polymerization reactions to create materials with specific properties.
Radiation besides modify catalyst surfaces, sometimes enhance their activity or selectivity. Researchers explore these effects to develop more efficient catalytic systems for industrial applications.
Kinetic isotope effects in radioactive systems
Kinetic isotope effects occur when reaction rates change due to isotopic substitution. These effects provide valuable information about reaction mechanisms and transition states. In radioactive systems, kinetic isotope effects help scientists understand decay processes and develop more accurate dating methods.
The study of isotope fractionation — the uneven distribution of isotopes between substances — depend on kinetic principles. This phenomenon creates distinctive isotopic signatures that serve as fingerprints for various natural processes, from photosynthesis to geological formations.
Future directions in kinetics, catalysis, and radioactivity research
Current research at the frontiers of these fields promise exciting developments with far reach implications:
Computational approaches
Advanced computational methods progressively model complex kinetic systems and predict catalytic behavior. Quantum chemistry calculations can instantly simulate reaction pathways and catalyst substrate interactions with unprecedented accuracy. Machine learning algorithms identify patterns in experimental data, accelerate catalyst discovery and optimization.
Computational approaches besides improve our understanding of radioactive processes, especially in complex environments like nuclear reactors or geological repositories. These models help predict long term behavior of radioactive materials and design safer containment systems.
Nanoscale phenomena
At the nanoscale, materials oftentimes exhibit unique kinetic and catalytic properties. Nanoparticle catalysts often show enhance activity compare to their bulk counterparts due to higher surface to volume ratios and distinctive electronic structures. Researchers develop progressively sophisticated methods to control nanoparticle size, shape, and composition to optimize catalytic performance.
Nanoscale radiation detectors offer improve sensitivity and spatial resolution compare to conventional instruments. These advances enable more precise radiation measurements in applications from medical imaging to environmental monitoring.
Sustainable applications
Sustainable chemistry progressively draws on kinetic and catalytic principles to develop greener processes. Researchers design catalyst that work expeditiously under ambient conditions, reduce energy requirements for chemical transformations.Biocatalystss — use enzymes for industrial processes — offer extremely selective reactions under mild conditions.
In the energy sector, advanced nuclear reactor designs aim to improve safety and efficiency while reduce waste production. Small modular reactors and generation iv designs incorporate passive safety features base on fundamental kinetic principles.
Conclusion
Kinetics, catalysis, and radioactivity represent fundamental scientific concepts with profound implications for our understanding of the natural world and our technological capabilities. From the microscopic movements of molecules to the transformations of atomic nuclei, these phenomena underlie countless processes essential to modern life.
As research continue to advance our understanding of these fields, new applications emerge that address press challenges in energy, medicine, environmental protection, and materials science. The intersections between these disciplines create peculiarly fertile ground for innovation, highlight the interconnected nature of scientific knowledge.
For students, researchers, and curious minds, these scientific domains offer rich opportunities for exploration and discovery. By understand the principles of kinetics, catalysis, and radioactivity, we gain deeper insights into the dynamic processes that shape our world and the tools to harness these phenomena for human benefit.
MORE FROM jobsmatch4u.com